- Home
- Research It
- IPO Buzz
- Biocon Limited
Biocon Limited
Issue Summary
| Type | Public Issue100% Book Building | Shares on offer | 10 mn shares |
|---|---|---|---|
| Size | Rs 2.7 bn - 3.15 bn | Face Value | Rs 5 per share. |
| Price Band | Rs 270 to Rs 315 | Promoters post issue holding | 61.53% |
| Minimum subscription | 50 shares | Promoters | Kiran Mazumdar Shaw, Glentec International |
| Listing | BSE & NSE | Lead Managers | DSP Merrill Lynch, Kotak Mahindra Capital Co, HSBC Securities |
| Bid/Issue opens | March 11, 2004 | Bid/Issue closes | March 18, 2004 |
ISSUE STRUCTURE
| QIBs | Non-Institutional Investor | Retail Portion | |
|---|---|---|---|
| No. of shares | 6 milliion | 1.5 million | 2.5 million |
| % offered from Net public offer | 60% | Minimum 15% | Minimum 25% |
| Minimum Bid/Application size | more than Rs 50,001 | more than Rs 50,001 | 50 equity shares |
| In multiples of | 50 shares | 50 shares | 50 shares |
| Maximum Bid/Application size | Not exceeding the issue size | Not exceeding the issue size | Not exceeding Rs 50,000 |
COMPANY BACKGROUND
Biocon is India's largest Biotechnology company with presence in biopharmaceuticals, enzymes, custom research and clinical research. It was established in the year 1978 to form a joint venture with Biocon Biochemicals Limited, an Ireland based company, to manufacture and export certain enzymes for the brewing industry. Unilever eventually acquired Biocon Biochemicals. The company came into its current form in 1999 when the promoters of company exercised their right of first refusal to purchase Unilever's interest in Biocon.
BUSINESS
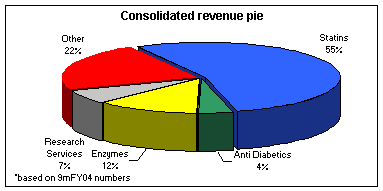
The major source of revenue for Biocon is Statins, which constituted 55% of its consolidated revenues in the first nine months of FY04. Statins inhibits the excess production of bad cholesterol in the liver. Biocon is also into manufacturing API's for immunosuppressants, anti diabetics, neutraceuticals, and other biopharma products. Enzymes also form a substantial portion of revenue for the company, though its share in revenue has come down in last few years. The basic business is based on fermentation process, which requires highly technological skills. Statins (Lovastatin, Simvastin, Pravastatin, and Atorvastatin) are the main products of Biocon and constitute 67% of the company's revenues. Almost 82% of the statins made by the company are exported. Biocon has patented process relating to manufacture of lovastatin and simvastatin. These drugs are cholesterol-reducing molecules, which belong to cardiovascular therapeutic segment, one of the fastest growing segments in pharma industry. The total market size of statins is about US$ 22 bn and is likely to grow at a rate of 18% in FY05. Presence of the company in this high growth segment is the key to its future growth.
Biocon also has patented a process technology PlaFactor for fermentation to manufacture immunosuppressants, another growth area where the company has strong focus. The revenue generated from this business is just 2.7% currently (on a consolidated basis), but future growth potential is encouraging, once the new manufacturing facilities get commissioned in FY06.
The company started as an enzyme manufacturer and diversified to biopharma, but enzymes still form a substantial portion of its revenues. Though the share of enzymes in revenue has come down over the years from 30% in FY01 to 12% in 9mFY04, the business has clocked a decent 15% CAGR in the last three years.
Another focus area for the company has been anti-diabetics. Biocon is launching an indigenously developed human insulin product (Insugen) in the Indian market by June 2004 under its own brand name. This will be its first product in Indian markets. The company has also signed an agreement with Bristol-Myers Squibb to supply human insulin in bulk form to markets like the US. There are several other products in the company's portfolio in anti diabetic segment mostly as API's in domestic and other unregulated markets. Biocon is looking at ways to supply these API's to US markets subject to approval of regulatory authorities.
The company has also established facilities for custom research and clinical research services. Syngene, a 99.99% subsidiary of the company, designs and manages research projects for multinational pharmaceuticals and biotechnology companies. The revenues from this subsidiary stood at Rs 258 m in 9mFY04, a growth of 31% on an annualised basis. This business has a good growth prospects considering the fact that India can be a hub for contract research going forward. Currently, this subsidiary accounts for 6.5% of consolidated revenues.
In clinical research, Biocon's other 100% subsidiary - Clinigene, has been established to carry out Phase I, Phase II and Phase III clinical trials. It is also establishing a disease profile database focused on India for further studies in clinical research.
Promoters
Biocon has been promoted by Ms. Kiran Mazumdar Shaw, her husband Mr. John Shaw and Glentec International, an investment firm owned by Mr. John Shaw. Post issue promoters will hold 61.53% of shares in the company compared to 68.38% currently. Mrs. Shaw is a first generation entrepreneur and is considered a leading light in the Indian biotechnology industry.
Objects of the Issue
The company intends to utilise the funds raised towards its expansion plans and funding of new projects. The proceeds will be used for setting up new facilities to augment the capacities for submerged fermentation and chemical synthesis operations. The company expects the expansion plan to cost Rs 4.1 bn and any further requirements would be met through internal accruals.
Sector
The global pharmaceutical industry had a size of US$ 401 bn in 2002 and over the period 2000-2002, the industry has clocked a CAGR of 12%. In terms of market share, the share of the North American region stands at nearly 51%. Europe is the next largest, accounting for 25% of total global sales. The table below indicates region wise sales break-up globally. It also indicates the growth in the respective regions.
| Region | 2000 | 2001 | 2002 | CAGR 2000-2002 |
|---|---|---|---|---|
| North America | 153 | 182 | 204 | 15.4% |
| Europe | 76 | 88 | 102 | 19.6% |
| Japan | 52 | 48 | 47 | -4.6% |
| Rest of the world | 38 | 47 | 48 | 27.9% |
| Total | 317 | 364 | 401 | 12.4% |
The global pharmaceutical markets can be divided mainly on the basis of regulated and unregulated/semi-regulated markets. North America and western European countries are regions that fall in the regulated market category. Other markets are mostly unregulated or semi-regulated. The regulated markets are characterized by a high degree of intellectual property right protection. That means that both product as well as process patents are recognized in these regions and are implemented very stringently.
As far as the unregulated/semi-regulated markets like India are concerned, the entry barriers are minimal due to low regulatory barriers with respect to intellectual property rights (IPRs). These markets do not recognize product patents and hence are a huge market for copied variants of drugs that have been patented in regulated markets. India is a semi-regulated market. The Indian pharma market is worth US$ 5 bn and has been growing at a rate of 8% over the last 3-4 years.
Due to the lack of IPRs, Indian companies have become very adept at reverse engineering and have managed to make copycat versions of already patented drugs. This has led to a situation where these companies have, through reverse engineering managed to get a toehold in the global generic pharmaceutical market. The generic pharmaceutical market mainly refers to the market for drugs whose patents have expired or have been invalidated in the regulated markets. The global generics market is estimated to be at US$ 38 bn.
REASONS TO APPLY
- Market potential: Statins (cholesterol inhibitors), which are essentially bulk drugs, are the largest contributors to Biocon's revenues (55%). This segment has big growth potential, as Biocon is essentially catering to the regulated markets like the US and Europe as far as its statins business is concerned. So far it seems to have only scratched the surface of this segment. The table below shows the market size for the various statins the company produces and also the timetable for the expiry of their patents in various markets. The table indicates the potential market for Biocon over the next 7-8 years. Biocon is well placed to tap this market in the future as it already has strong presence in the European markets for Lovastatin and Simvastatin. It also is one of the largest suppliers of statins in the regulated US markets. The potential for growth can be gauged from the fact that till FY02, Biocon's revenues from Simvastatin were only Rs 150 m, as it was a patented product. In FY03, when its patent expired in UK and Germany, Simvastin revenues shot up to Rs 681 m. In 9mFY04 so far, revenues from this statin stood at over Rs 1.4 bn.
- Biocon's R&D expertise: The company is operating in bulk drug segments that require a high level of R&D. Biocon's product portfolio is targeted towards the regulated markets and hence it is imperative that the company had to develop non-infringing process for the production of its various products. The fact that the company has a wide range of products, both biopharmaceutical as well as enzymes, indicates that Biocon has developed significant R&D capabilities that have enabled it to create non-infringing patents. An example of the company's R&D capabilities is the fact that the company has a patented hybrid fermentation process called 'PlaFactor' that has been developed to manufacture immunosuppressants. The company also has expertise in synthetic chemistry that it has leveraged to further expand its product offerings. An indication of the company's commitment to R&D is highlighted in the graph below. R&D expenses, as a percentage of total sales are relatively high indicating the focus on the same.
- Manufacturing capabilities: Biocon also has manufacturing capabilities that have been approved by various agencies including the US FDA and the European EDQM. The company continues to seek approval for its various facilities from these agencies based on the expiry of patents in various regulated markets like the US and Europe. In order to cater to the current shortfall in supply and any future demand, the company is targeting a very aggressive capacity expansion program. The company also benefits from the fact that the same manufacturing facility can be used to produce different products. For example, the company is able to produce lovastatin, pravastatin and compactin (among others) from the same submerged fermentation facility. This will help the company change the product mix based on the prevailing demand in the market.
Biocon has already commenced Rs 4.1 bn capacity expansion drive mainly to further augment its existing capacity of various products. The table above gives us an indication regarding the nature of expenditure and the segments it is going into. Higher capacity for its various products is likely to aid the company significantly in the future as it will be able to cater to expected demand as patents expire in the regulated markets.Capital expenditure Rs m For statin production 4,070 For human insulin production 22 For immunosuppressants 200 For research facilities 300 Total 4,592 - Subsidiary strengths: Biocon has also established facilities for custom research and clinical research services. Syngene - a 99.99% subsidiary of the company designs and manages research projects for multinational pharmaceuticals and biotechnology companies. Its revenues stood at Rs 258 m in 9mFY04 - an annualised growth rate of 31%. In clinical research, Biocon has established a wholly owned subsidiary - Clinigene, to carry out Phase I, Phase II and Phase III clinical trials. Considering that India could emerge as a hub for contract research in future these subsidiaries could contribute significantly to the company's growth going forward.
| Statin | Total market in US$ mn (Year ended Sept 2003) |
Patent expiry |
|---|---|---|
| Lovastatin | 411 | Expired |
| Simvastatin | 7,024 | US-2006, UK and Germany- Expired, France-2005 |
| Pravastatin | 4,091 | US-2006, UK and Germany-2004, France-2006 |
| Atorvastatin | 9,986 | US-2011, UK and Germany-2010 |
REASONS NOT TO APPLY
- Statin dependent: Around 55% of Biocon's (consolidated) revenue comes from statins, which is subject to high competition. Once these statins go off patent there will be intense competition in the markets for the same. The company is unlikely to be able to maintain such high margins in the future. Also, Biocon has to regularly update its status on the statins. The technology in these field changes very fast and this can make the products of the company obsolete. For example, a higher order statin might erode the market for lower order statin in which company may be currently focused on. Also, a rise of new treatment method in that therapeutic category can impact the overall topline and bottomline of the company.
- Limited clientele: The biopharmaceutical business contributes nearly 80% to the company's consolidated revenues. Within this business, Biocon's revenue profile is highly concentrated. Currently, Biocon's top 10 customers contribute about 46% to its biopharmaceutical revenues, with the top two customers contributing 24%. Consequently, there is a huge risk to its revenue profile even if its loses one big customer. There is also a risk that the company's bulk drug customers, who are generic drug producers, may lose their necessary regulatory approvals, which might lead to termination of contracts.
- Regulatory risk: Any pharmaceutical company is subject to regulatory changes, which may have adverse impact on its profitability. Biocon is focusing on exports market, US and Europe in particular, for its future growth where the rules and regulations for pharmaceutical industry are quiet tough. Since the business of the company deals with human health and life, entering into new product segment requires a lot of regulatory clearances from different government agencies in each country of operation. Even post the drug's introduction into the market, it is subject to continued review. Any discovery of inefficacy, or any previously unknown problems with safety of drugs may result in restrictions, which are detrimental to revenue and profit growth.
- Competitive threat: Currently, Biocon is the largest player in Biotech business in India. Internationally, it has a significant cost advantage, but once players from countries like China come into the market, it may not be possible for the company to operate at high margins. Also, once the drugs that are off patent, their prices reduce significantly due to significant increase in number of players, which can also impact the profitability. Also, Biocon is relatively small in comparison to big international players who have significant financial strength to withstand pressure if competition intensifies. In custom research business too, it faces challenges because there are a number of players who have entered the business or are planning to enter.
FINANCIAL PERFORMANCE
| (Rs m) | FY01 | FY02 | FY03 | 9mFY04 |
|---|---|---|---|---|
| Income from Operations | 1,234 | 1,616 | 2,548 | 3,718 |
| Revenue Growth | 66.3% | 31.0% | 57.7% | 94%* |
| Other Income | 2 | 34 | 8 | 7 |
| Total Income (%) | 1,236 | 1,650 | 2,556 | 3,725 |
| Expenditure | 917 | 1,252 | 1,910 | 2,527 |
| Operating Profit | 317 | 364 | 638 | 1,191 |
| Operating Profit Margin | 25.7% | 22.5% | 25.0% | 32% |
| Depreciation | 73 | 78 | 120 | 101 |
| Interest | 45 | 47 | 49 | 12 |
| Profit Before Tax | 199 | 240 | 469 | 1,077 |
| Tax | 38 | 71 | 119 | 218 |
| Net Profit | 161 | 169 | 350 | 859 |
| Net Profit Margin (%) | 13.2% | 12.3% | 14.0% | 23.2% |
| Diluted Earnings per share (Rs) | 2 | 2.8 | 3.9 | 9.6 |
| Key Ratios | ||||
| RONW | 26% | 25% | 28% | 41% |
| ROA | 11.2% | 10.3% | 13.0% | 21.3% |
Shareholding
| Category | Pre-Offer | Post-Offer |
|---|---|---|
| Promoters | 68.4% | 61.5% |
| Relatives of Promoters | 0.8% | 0.7% |
| Holding of Directors | 0.3% | 0.4% |
| Employees | 7.8% | 7.0% |
| Venture Capital Funds | 12.4% | 11.1% |
| Others | 10.3% | 9.3% |
| Alloted to the pursuants of Public Offer | - | 10.0% |
| Total | 100% | 100% |
Valuations and comments
At Rs 315 the stock of the company will be at 27.3x FY04 earnings. At this price it seems that the stock is priced aggressively as of now. Since, there are no biotech companies with such a business model it is hard to compare. But, looking at the business fundamentals and the growth expected in the biotech industry this stock can be a good investment opportunity for investors looking to invest for long term for the period of 3-5 years. Overall positives seems to outweigh negatives from a longer term perspective.
Your feedback is important to us. Please Write to us
Disclaimer:
We would like to inform our readers that this IPO note is just a one-time view on the company and in no way implies that there will be regular coverage on the company's performance or any other development. Should we decide to bring the company under research coverage in the future, it will be available exclusively to subscribers of the respective subscription.
|
| |

