- Home
- Views On News
- May 25, 2021 - Cipla's New Covid-19 Drug to Be Sold at Rs 59,750 Per Dose
Cipla's New Covid-19 Drug to Be Sold at Rs 59,750 Per Dose

Swiss multinational drug firm Roche's antibody cocktail (Casrivimab and Imdevimab) is now available in India, its marketing partner here Cipla said on Monday.
An antibody cocktail is a mix of two or more unique biological drugs that act like human antibodies to help fight off infection.
The first batch of this therapy is now available, the firm said, and the second batch will be available by mid-June.
In total they can potentially benefit 2 lakh patients as each of the 1 lakh packs that will be available in India offer treatment for two patients.
The price for each patient dose (a combined dose of 1,200 mg that is 600 mg of Casirivimab and 600 mg of Imdevimab) will be Rs 59,750 inclusive of all taxes.
The maximum retail price for the multi-dose pack (each pack can treat two patients) is Rs 1.2 lakh inclusive of all taxes.
Cipla will distribute the product in India through leading hospitals and Covid treatment centres.
The Central Drugs Standards Control Organisation (CDSCO) had recently granted emergency use authorisation (EUA) for the antibody cocktail (Casirivimab and Imdevimab) in India.
It has also received a EUA in the US and several EU countries.
V Simpson Emmanuel, MD and CEO, Roche Pharma India, said,
- Roche is deeply committed to supporting the ongoing efforts to combat the Covid-19 pandemic, mitigate the deadly second wave and save lives.
We are optimistic that the availability of antibody cocktail (Casirivimab and Imdevimab) in India can help in minimising hospitalisation, ease the burden on healthcare systems and play a key role in treatment of high-risk patients before their condition worsens.
The antibody cocktail (Casirivimab and Imdevimab) is to be administered for the treatment of mild to moderate coronavirus disease 2019 (Covid-19) in adults and pediatric patients (12 years of age or older, weighing at least 40 kg) who are confirmed to be infected with SARS-COV2 and who are at high risk of developing severe Covid-19 disease and do not require oxygen.
"It has been shown to help these high-risk patients before their condition worsens, reducing the risk of hospitalisation and fatality by 70% and shortening the duration of symptoms by four days," the statement claimed.
An Overview on Cipla's Q4 Performance
Earlier this month, drug major Cipla posted 68% rise in consolidated net profit at Rs 4.1 bn for the fourth quarter ended 31 March 2021.
The Mumbai-based firm had reported a net profit of Rs 2.5 bn in January-March 2019-20.
For the entire 2020-21 fiscal year, the drug maker reported a consolidated net profit of Rs 24 bn as against Rs 15.5 bn in the previous year.
On a quarter-on-quarter (QoQ) basis the net profit stood at Rs 4.1 bn, down 44.8%, as compared to Rs 7.5 bn in the December 2020 quarter.
The company's total revenue from operations rose to Rs 46.1 bn, up 5%, compared to Rs 43.8 bn in the same period of 2019-20.
Total revenue from operations for the last fiscal year rose to Rs 191.6 bn as against Rs 171.3 bn in 2019-20.
The company's consolidated earnings before interest, tax, depreciation, and amortisation (EBITDA) in the quarter rose 22% year on year (YoY) to Rs 8 bn with margins expanding 239 basis points (bps) to 17.3%.
For the full fiscal year 2021, EBITDA stood at Rs 43 bn, up 33%, compared to Rs 32.3 bn reported in the previous financial year.
The board of directors of the company also recommended a final dividend of Rs 5 per equity share (face value Rs 2 each) for the financial year ended 31 March 2021.
Cipla Inks Pact With Eli Lilly To Make, Sell Covid-19 Medicine
On 10 May 2021, Cipla reported that it has entered into a pact with US-based Eli Lilly and company to manufacture and produce Baricitinib in the country for the treatment of Covid-19.
The Mumbai-based company has signed a royalty-free, non-exclusive voluntary licensing agreement with Eli Lilly for Baricitinib.
Baricitinib has already received restricted emergency use approval by the central drugs standard control organisation (CDSCO), Ministry of Health, India, for use in combination with remdesivir for the treatment of suspected or laboratory-confirmed Covid-19 in hospitalised adults requiring supplemental oxygen, invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO).
This collaboration is a step further in the company's efforts to enhance access to critical treatments for patients affected by the pandemic, Cipla said in a statement.
The company will leverage its extensive distribution footprint to make this therapy accessible to more patients and markets, it added.

Equitymaster's View on the Pharma Sector
We reached out to Tanushree Banerjee, Co-Head of Research at Equitymaster, and editor of the premium stock recommendation service StockSelect, for her view on the pharma sector.
Here's what she has to say...
- The second Covid wave has given a new lease of life to pharma stocks. As the sector continues to invest in capacities for new drugs, the profitability will depend on the companies' ability to seek USFDA approval for the plants.
Or their ability to tie up with MNC pharma for producing variants of their vaccine.
How the Stock Markets Reacted to Cipla's News Today
Shares of Cipla opened the day at Rs 944 on the BSE and Rs 939.7 on the NSE.
At the time of writing, the company's shares were trading up by 0.7% on the BSE.
At its current price, it is trading at a P/E of 21.6.
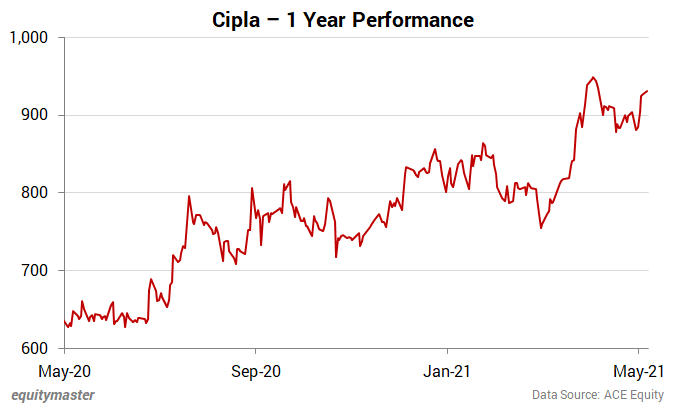
The share touched its 52-week high of Rs 966 and 52-week low of Rs 618.9 on 20 April 2021 and 12 June 2020, respectively.
Over the last 30 days, the Cipla share price is down 1.4%. Over the last one year, the company's share price is up 51%.
About Cipla
Cipla is one of the leading pharmaceutical companies in India. It was incorporated in the year 1935 with the name Chemical Industrial & Pharmaceutical Laboratories Limited.
The company focuses on development of new formulations and has a wide range of pharmaceutical products.
Khwaja Abdul Hamied the founder of Cipla gave the company all his patent and proprietary formulas for several drugs and medicines without charging any royalty.
Cipla is also a global pharmaceutical company focused on agile and sustainable growth, complex generics, and deepening portfolio in India, South Africa, North America, and key regulated and emerging markets.
Its strengths in the respiratory, anti-retroviral, urology, cardiology, anti-infective, and CNS segments are well-known.
The company has 46 manufacturing sites around the world produce 50+ dosage forms and 1,500+ products using cutting-edge technology to cater 80+ markets.
Cipla is ranked 3rd largest in pharma in India (IQVIA MAT March-2021), 3rd largest in the pharma private market in South Africa (IQVIA MAT March-2021), and is among the most dispensed generic players in the U.S.
For more details about the company, you can have a look at Cipla's factsheet and quarterly results on our website.
You can also compare Cipla with its peers:
To know what's moving the Indian stock markets today, check out the most recent share market updates here.


Equitymaster requests your view! Post a comment on "Cipla's New Covid-19 Drug to Be Sold at Rs 59,750 Per Dose". Click here!
Comments are moderated by Equitymaster, in accordance with the Terms of Use, and may not appear
on this article until they have been reviewed and deemed appropriate for posting.
In the meantime, you may want to share this article with your friends!