- Home
- Views On News
- Jul 8, 2021 - Bajaj Healthcare Rallies 11% on DRDO Nod to Manufacture Oral Covid Drug
Bajaj Healthcare Rallies 11% on DRDO Nod to Manufacture Oral Covid Drug

Shares of Bajaj Healthcare surged 11% in early trade today after the company received license from defence research and development organisation (DRDO) to manufacture and market '2-Deoxy-D-Glucose' (2-DG).
This drug is used as a treatment of Covid-19, which is given as an oral intake.
The 2-DG Covid-19 control and treatment drug has been developed by the DRDO laboratory's Institute of Nuclear Medicine and Allied Sciences (INMAS), in collaboration with the pharma major Dr Reddy's Laboratories.
It has already received emergency approval from the drugs controller general of India (DCGI) for use on Covid-19 patients in the country.
The drug comes in powder form and retails at Rs 990 per sachet.
2-DG helps in the faster recovery of hospitalised patients and reduces supplemental oxygen dependence.
The drug works by selectively accumulating in the virus-infected cells and prevents virus growth by stopping viral synthesis and energy production.
It can be administered only upon prescription and under the supervision of a qualified physician to hospitalised moderate to severe Covid-19 patients as an adjunct therapy to the existing standard of care.
Commenting on license received from DRDO for 2-Deoxy-D-Glucose, Anil Jain, Joint Managing Director, Bajaj Healthcare said,
- We hope the availability of an effective treatment such as 2-DG will considerably ease the pressure and offer patients much needed and timely therapy option.
Most patients ailing from moderate to severe symptoms can benefit from the use of Deoxy-D-Glucose.
Bajaj Healthcare seeks compulsory license for Covid-19 drug
On 28 June 2021, Drugmaker Bajaj Healthcare requested the Indian patent office to grant a compulsory license to make Baricitinib, an Eli Lilly drug which has shown promise in treating Covid-19.
Bajaj Healthcare, which makes Covid drugs like favipiravir and ivermectin, has sought permission to make both the active pharmaceutical ingredient (API) and the formulation of Baricitinib.
The company approached Eli Lilly on two occasions, to sign the voluntary license for manufacturing and supply of Covid-19 drug Baricitinib.
Company's board approves listing on NSE
On Monday, Bajaj Healthcare has informed to the exchange regarding listing of equity shares on National Stock Exchange (NSE).
The board of directors of the company has considered and approved the listing of 13.7 m equity shares of Rs 10/- each of the company on NSE.
Key financial highlights for the March 2021 quarter
The company reported 11.5% year on year (YoY) rise in consolidated net revenues for the January - March quarter at Rs 1.3 bn.
Net profits in the quarter four were up 69.4% at Rs 212.8 m on the back of higher sales and lower raw material costs which helped the growth in profits.
In terms of verticals, the bulk drugs vertical reported 13% higher sales at Rs 1.3 bn while the formulations verticals saw its revenues falling by 17% at Rs 49.4 m.
The earnings before interest, depreciation, and tax (EBIDTA) contribution was sharply higher for the bulk drugs business and flat for formulations despite the fall in sales in the formulations vertical.
Equitymaster's view on the pharma sector
We reached out to Tanushree Banerjee, Co-Head of Research at Equitymaster, and editor of the premium stock recommendation service StockSelect, for her view on the pharma sector.
Here's what she has to say...
- The second Covid wave has given a new lease of life to pharma stocks. As the sector continues to invest in capacities for new drugs, the profitability will depend on the companies' ability to seek USFDA approval for the plants.
Or their ability to tie up with MNC pharma for producing variants of their vaccine.
How the stock markets reacted to Bajaj Healthcare
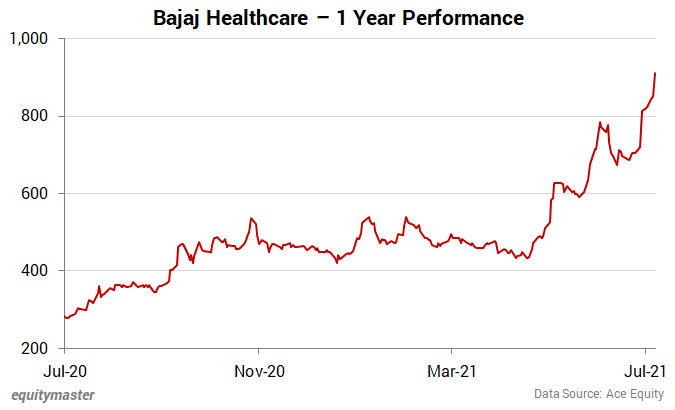
Shares of Bajaj Healthcare opened the day at Rs 950 on the BSE.
Its share price closed at Rs 978.3 (up 7.4%) on the BSE.
At its current price, it is trading at a P/E of 16.2.
The share touched its 52-week high of Rs 1,009.8 and 52-week low of Rs 277 on 8 July 2021 and 8 July 2020, respectively.
Over the last 30 days, the Bajaj Healthcare share price is up 28.4%. Over the last one year, the company's share price is up 243.6%.
About Bajaj Healthcare
Bajaj Healthcare is a leading manufacturer of APIs, intermediates and formulations established in the year 1993.
It specialises in manufacturing of amino acids, intermediates, API, formulations, and nutraceuticals.
The company has state-of-art manufacturing facilities, of which 6 units are dedicated to APIs, 2 units to intermediates and 1 unit for formulation.
These facilities are designed to meet the requirements of both advanced as well as emerging market opportunities.
Bajaj Healthcare has a strong presence globally in countries like Europe, USA, Australia, Africa, Middle East, and South America.
For more details about the company, you can have a look at Bajaj Healthcare factsheet and quarterly results on our website.
You can also compare Bajaj Healthcare with its peers.
Bajaj Healthcare vs DR Reddys Lab
To know what's moving the Indian stock markets today, check out the most recent share market updates here.
Disclaimer: This article is for information purposes only. It is not a stock recommendation and should not be treated as such. Learn more about our recommendation services here...


Equitymaster requests your view! Post a comment on "Bajaj Healthcare Rallies 11% on DRDO Nod to Manufacture Oral Covid Drug". Click here!
Comments are moderated by Equitymaster, in accordance with the Terms of Use, and may not appear
on this article until they have been reviewed and deemed appropriate for posting.
In the meantime, you may want to share this article with your friends!